- ABOUT SOMATULINE DEPOT
- How Can it Help?
SOMATULINE® DEPOT IS PROVEN TO HELP PATIENTS WITH GEP-NETs
Slowed the growth of GEP‑NETs in patients whose disease has spread or cannot be removed by surgery
Time to disease progression or death in a clinical study
IN A CLINICAL STUDY, SOMATULINE DEPOT REDUCED THE RISK OF DISEASE PROGRESSION OR DEATH BY 53% VERSUS PLACEBO
AT 22 MONTHS, CANCER DID NOT PROGRESS IN MORE THAN HALF OF PATIENTS TAKING SOMATULINE DEPOT (EVENTS OCCURRED IN 32 OUT OF 101 PATIENTS [31.7%] VERSUS 60 OUT OF 103 PATIENTS [58.3%] WITH PLACEBO)
FOR THOSE TAKING PLACEBO, IT TOOK 16.6 MONTHS UNTIL CANCER PROGRESSED IN HALF OF PATIENTS
Somatuline Depot was studied for nearly 2 years in 204 adult patients with various types of GEP-NETs that had spread or could not be removed by surgery. In some patients, the cancer started in their pancreas. In others, it started in a different place, such as their intestinal tract, which includes the colon.
Patients in the study were divided into two groups, which received either Somatuline Depot 120 mg or placebo by deep subcutaneous injection every 4 weeks. The primary goal of this study was to determine if Somatuline Depot improved progression-free survival, which is the amount of time it took for the disease to progress.


*Assessed by a central independent radiological review in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0.

Reduced the number of rescue medication days for carcinoid syndrome

SOMATULINE DEPOT HELPED REDUCE THE NUMBER OF DAYS OF RESCUE MEDICATION PATIENTS MAY NEED
Rescue medications are short-acting medicines used to lessen symptoms of carcinoid syndrome, including diarrhea and flushing.
In other words, patients on Somatuline Depot experienced 15% fewer days on rescue medication compared to patients on placebo (34% of days on rescue medication for patients on Somatuline Depot versus 49% of days on rescue medication for patients on placebo).
With Somatuline Depot, patients experienced approximately 4.4 fewer days of rescue therapy per month, or around 53 fewer days per year.
FDA-approved to treat adults with carcinoid syndrome to reduce the need to use short-acting somatostatin medicine
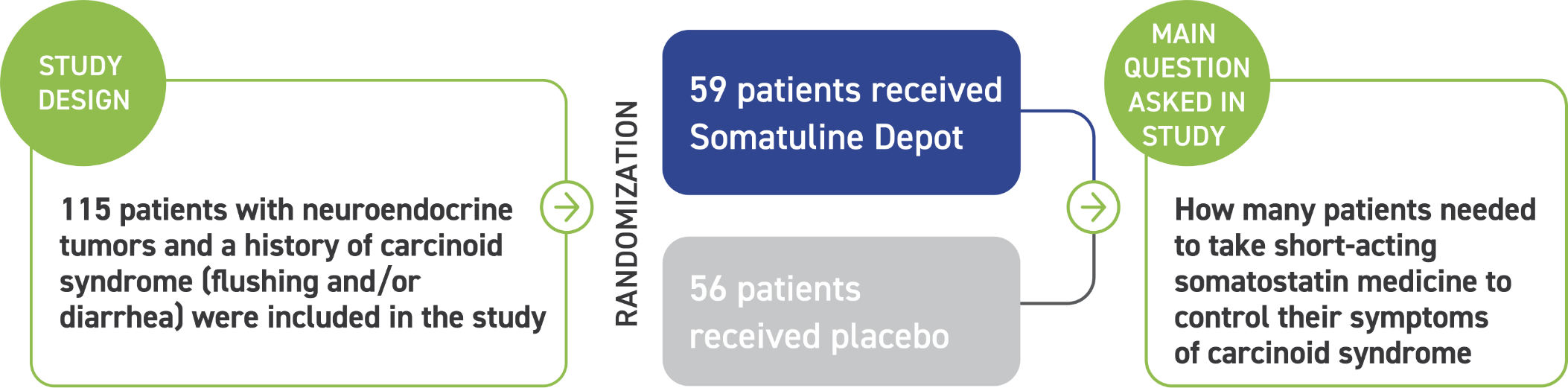
Somatuline Depot was studied for 16 weeks in 115 patients with confirmed NETs and a history of carcinoid syndrome (flushing and/or diarrhea) to reduce the need for short-acting somatostatin medicine.
Patients were divided into two groups, which received either Somatuline Depot 120 mg or placebo by deep subcutaneous injection every 4 weeks. Many patients were living with carcinoid syndrome as a result of being diagnosed with NETs for less than a year before starting treatment.
 See the common side effects with Somatuline Depot
See the common side effects with Somatuline Depot 
